This method details how to test for total VDK in beer, not to be mistaken as total diacetyl in beer. Total VDK includes the precursor and the two VDKs found in beer, 2,3-butanedione (diacetyl) and 2,3-pentandione. Diacetyl specifically is considered an off-attribute in beer. High levels of diacetyl are responsible for buttery and butterscotch-like flavors that indicate unhealthy or incomplete fermentation. This is a colorimetric method which requires the distillation of an unknown sample and a spectrophotometer. A calibration curve of known standards is create and a linear regression of response (absorbance) vs. concentration (total ppb VDK) is determined. Unknown samples and their total VDK concentration can then be determined.
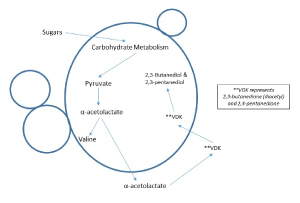
Figure 1.
Metabolic pathways of VDK formation by brewer's yeast - Click to Enlarge
Diacetyl (2,3-butanedione) and 2,3-pentanedione are compounds that can be found in beer and wort that present as a buttery or butterscotch flavor. They are commonly referred to as VDK (for vicinal diketones). VDK compounds can be a desired or required flavor in some styles of beer, but they are considered an off flavor at moderate or elevated concentrations. Diacetyl and 2,3-pentanedione are produced from precursor compounds created as intermediates during yeast’s synthesis of the amino acid valine. The yeast often overproduce these intermediates, causing them to be expelled into solution where they spontaneously undergo the reaction into VDK compounds. With time VDK compounds are taken back into the yeast cellsand consumed for continued metabolism or they may be enzymatically converted into compounds that have much higher flavor thresholds than diacetyl and 2,3-pentanedione.If the precursors (alpha-acetolactate and alpha-acetohydroxybutyrate) are not completely converted during fermentation or maturation to diacetyl and 2,3-pentanedione, thereby allowing yeast to consume them, then they will survive into the beer and progressively degrade in the final package (Inoue and Yamato, 1971).

Figure 2. Calibration curve standards post color reaction - Click to Enlarge
The VDK broad spectrum method originated in 1936 when Maxwell Barritt at the University of Liverpool modified an 1898 bacterial identification method called the Voges-Proskauer (V-P) test which was used to detect enteric bacteria by a color change reaction in broth culture (Barritt, 1936). Barritt found that by slightly modifying the V-P reaction, he could characterize a chemically specific and sensitive method for the detection of diacetyl (2,3-butanedione) by bacteria. The addition of α-Naphthol at the beginning of the reaction helped with intensifying the color change of the reaction between potassium hydroxide (KOH) and diacetyl. The addition of creatine to the KOH solution helped with increased sensitivity of detection with the color change reaction with diacetyl.
While Barritt’s modified test was used to see color changes related to the presence of bacteria in cultures, the same reaction can be used to quantify the yeast derived VDK content in beer. The ASBC first published their method for VDK testing in beer via the broad spectrum VDK method in 1964.
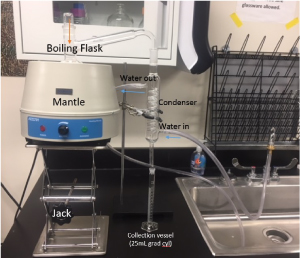
Figure 3.
An example distillation set up - Click to Enlarge
VDK levels can be determined in beer following filtration, degassing, and distillation of the beer sample, and initiation of a color change reaction after addition of α-Naphthol and a KOH-creatine solutions to the beer distillate. After shaking the mixture and oxidizing the strong base KOH with atmospheric oxygen exposure, the VDK in the distillate will react to turn a pink color with the intensity varying from light pink to bright magenta depending on the amount of VDK present. The level of color change for each distilled beer sample can then be measured by absorbance values on a UV-Vis spectrophotometer at 530nm, and compared to a standard curve from known concentrations of VDK to obtain a concentration of VDK in parts per billion (ppb).
The ability to quantify levels of VDK using the broad spectrum method can allow breweries to track levels in their beer to understand whether their beer has completed healthy fermentation. Brewers can then make informed decisions on whether or not to initiate the next steps of the cellaring process or to allow the beer additional fermentation time to clean up VDK.
Green Chemistry
|
Green Action |
Principle |
Benefit |
Degas by sonication or agitation
|

Prevent Waste | Eliminates costs and waste associated with degassing by filter paper. paper filter. |
| Compost Filter Paper |

Atom Economy | Minimizes filter paper waste. |
| Use a Bottle Top Dispenser for Reagents |
Safer Solvents | Safer liquid transfer. Reduce single-use plastics. |

Prevent Accidents
|

Prevent Waste |
| Use a water pump or recirculating chiller during distillation |

Atom Economy | Prevent water waste. Keep water cold by cyling ice packs. |

Prevent Waste |
| Collect VDK reaction waste in a class amber bottle |

Prevent Waste | The liquid waste from the reaction of beer with reagents is a strong base and must be disposed of as hazardous waste. |
Icons provided by TheNounProject from creator iconsmind.com
- When filtering α-Naphthol, make sure all activated charcoal has been removed to prevent interference with the absorbance measurement.
- Re-calibrate curve once a week or when new reagents are made.
- To filter and de-gas beer sample before distillation, use Grade 1 qualitative filter paper to remove CO2 and particles of hop/malt/yeast to halt fermentation of the sample.
- Do not let the filtered sample remain exposed to the air for a long period of time. This will lead to the volatilization of the compounds of interest and therefore a false low measurement. Distill sample as soon as possible after filtration is complete.
- When preparing the standard curve filling the 10mL volumetric flask to precisely 5.0 mL is not important at this step because the volumetric flask will be topped off to precisely 10.0 mL with reagent water at the end of the reagent additions.
- Use 1-2 drops of anti-foaming agent in distillation flask to prevent boiling over beer into distillate.
- Turn on spectrophotometer and allow lamp to warm up for at least 10 minutes.
- The color change reaction involves the addition of reagents in this specific order. First add the distillate and then α-Naphthol must be added before the KOH-creatine solution, otherwise the reaction may be weak or produce a false-negative reaction.
- Unit Conversion Tip: 1 mg/L = 1 ppm = 1000 ppb
- Reaction liquid should be collected in a compatible container and disposed of properly. The VDK chemical waste stream is a strong base due to the 40%KOH reagent and the α-Naphthol is toxic to aquatic life. Connect with your local hazardous waste facility to determine what measures they require for disposal.
Explanation of the calibration curve calculation:
A major point of confusion for many users of this method is calculating the standards curve. Care must be taken to take into account the concentration 4x factor of the distillation, as well as the fact that distillate is being diluted by a factor of 2 in the 10mL volumetric flask.
A standards curve should cover the range at which the method is expected to measure. For the purposes of measuring finished beer in the brewery laboratory, a range of 25-250 ppb of standards concentration is recommended.
An example procedure and calculation explanation is below. Depending on available glassware, dilutions may look different in your lab:
- Weigh 500 mg of 99% Diacetyl into a 100 mL volumetric flask half full with reagent water. Top off with reagent water to the mark. Cap and mix.
- Transfer 10.0 mL of reagent from step 1 into a 100 mL volumetric flask. Top off with reagent water to the mark. This 1:10 dilution will give you 100mL solution of 500 mg/L diacetyl. This is the ‘stock solution’ from the method.
- The 5 mg/L ‘working solution' is prepared by diluting 1 mL of stock solution into a 100 mL volumetric flask and filling to the mark.
- Unit Conversion Tip: 1 mg/L = 1ppm = 1000ppb
- To prepare a single
250 ppb standard with 5 mg/L working solution:
- First, calculate how much working solution (WS) is required for a 250 ppb calibration standard:
- The method begins with 100.0 mL of beer that is distilled to 25mL in the reaction flask. This is a concentration factor of 4 of Total VDK. A calibration standard that is equivalent to a beer with diacetyl concentration of 250 ppb will be 1000 ppb in the distillate.
- In the method, 5.0 mL of distillate is measured into a 10 mL volumetric flask. For the purposes of calculating standard concentration, use 5.0 mL of volume and multiply the desired standard concentration by 4.
-
C1
* V1 = C2
* V2
-
[WS]
* [Vol of WS] = [Standard]
* [Vol of Standard]
-
5000 ppb
* V1 = 1000 ppb
* 5mL
-
V1 =
(1000ppb
* 5mL)⁄5000ppb
-
V1 = 1.0mL
- To prepare a 250ppb calibration standard from a 5ppm diacetyl working solution, add 1.0 mL of working solution to a 10mL volumetric flask. Add water to approximately 5mL. Proceed with color formation reaction from method.
A Big Thanks To...
Max Kravitz, Robert Fulwiler, Krystin Norman & Katie Fromuth
