This method estimates the bitterness in wort and beer samples. Bitterness in beer originates from hop products added during the boiling of the wort, used in a dry-hop, post-fermentation or to adjust finished beer bitterness. This method uses the physical agitation of an organic non-polar solvent (isooctane), a strong acid (HCl), and a wort or beer sample to extract bittering compounds. The isooctane and aqueous beer layers are then separated by centrifugation and the absorbance of the upper solvent layer, containing all the extracted bittering compounds, is measured at 275 nm on a UV/VIS spectrophotometer. The absorbance is then used in the method calculation to determine Bitterness Units (BU). BU estimations of bitterness are commonly used to compare production runs of the beer as a way to measure quality, bitterness consistency and new products or hop varieties.
Iso-alpha acids are organic non-polar compounds formed during the kettle boil from the acids found in hops. This method’s extraction protocol utilizes the principle of “like dissolves like”. First, the beer sample is acidified with hydrochloric acid. The addition of hydrochloric acid reduces the solubility of the iso-alpha acids and many other hop bittering compounds and polyphenols in the polar aqueous wort/beer sample, facilitating the migration of the bittering compounds into the non-polar solvent layer.
The acidified mixture is shaken in a rocking motion, preferably using a horizontal, wrist-action shaker, to extract all the hop bitters from the aqueous layer. After extraction, the layers are separated by centrifugation into three layers: an upper isooctane solvent layer, the interface (emulsion) layer, and the acidified lower aqueous layer.
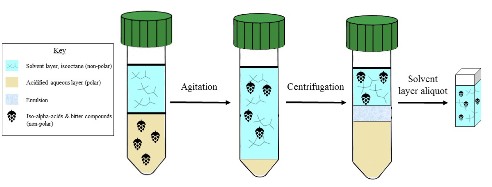
Figure 1. Diagram showing the three extraction layers and movement of compounds at each step of the BU extraction. Image credit: Katie Fromuth. Click to enlarge.
a.  b.
b.  c.
c. 
Figure 2. Photographs of BU extractions at each step, a) addition of reagents to conical tube, b) emulsion post agitation, and c) separation of the three phases after centrifugation. Photo credit: Katie Fromuth. Click to Enlarge.
Once separated, an appropriate volume (approximately 2.0 mL) of the isooctane layer, with no emulsion, is carefully pipetted to a spectrophotometer cuvette and absorbance of the sample is read at 275 nm.
The absorbance of the solvent layer at 275 nm is, in general, proportional to the concentration of the dissolved iso-alpha acids and other hop compounds in the original sample. The absorbance value from the spectrophotometer is converted into Bitterness Units (BU) by multiplying by a factor of 50.
The BU calculation will measure any substance that is soluble in isooctane and absorb at 275 nm and that are affected by UV light-scattering in lower hopped and highly hopped beers (Beer-Lambert Law). The BU method is commonly used in breweries as it provides a fast and reasonable measurement of bitterness levels in the finished beer for process control.
Repeatability and reproducibility coefficients of variation for the determination of bitterness unit (BU) levels in wort, ranging from 20 – 85 BU, using the spectrophotometric method ranged from 1.5 to 4.5% and 4.3 to 10.0%, respectively.
For dry hopped beers, the IAA spectrophotometric method (Beer 23F) was better at estimating the IAA levels determined by HPLC method. Therefore, Beer 23F may be a more appropriate method for assessing the perceived bitterness in dry hopped beers.
Green Chemistry
|
Green Action |
Principle |
Benefit |
| Purchase hydrochloric acid at method concentration (3N HCI). |
Safer Solvents | Diluting concentrated hydrochloric acid creates safety hazards. Always add acid to diluent to avoid the risk of spraying acid on lab technicians. |

Prevent Accidents
|
| Use the
Manual Isoctane Extraction: Reduced Solvent Technique. |

Atom Economy | Reduces hazardous waste produced by running the method by half. Labs should validate this method before adopting. |

Prevent Waste |
| Purchase bottle top dispensers for reagents. |

Prevent Accidents
| protect technicians from chemical hazards. |
| Avoid using octanol and Antifoam B. |

Prevent Waste | Removes the use of unnecessary chemicals. Well-degassed samples by agitation elimate the need for these reagents. |
| Switch to glass Pasteur pipettes for solvent transfer. | Reduces the use of single-use plastics |
| Use glass centrifuge tubes for reuse. | Reduces the use of single-use plastics. |
| Regenerate isooctane from method waste for reuse. | Reduces solvent waste and costs associated with hazardous waste disposal. This method is detailed in the
MBAA Technical Quarterly article, Volume 56. |
| Collect reagent waste for proper hazardous disposal. | Reagent waste is a strong acid and requires hazardous waste disposal. |
Icons provided by TheNounProject from creator iconsmind.com
Avoid Excess Foaming
- It is strongly suggested not to use octanol to break foam due to the toxicity to humans and aquatic life, and the availability of a non-toxic alternative (Antifoam).
- It is important to capture the foam as it contains iso-alpha-acids and other bittering compounds. Failure to do so could result in erroneous results.
Layer Separation and Emulsions
- Not all samples after agitation will form an emulsion.
- If the emulsion interface is too large after the first centrifugation to safely sample the isooctane layer without aspirating the emulsion, try gently tapping or inverting the BU tube, vent, then retighten the cap and centrifuge again. Repeat if necessary.
- The separation is sufficient so long as enough of the isooctane layer can be pipetted (approximately 2.0 ml) without including any of the emulsion mixture.
- Higher centrifuge speeds to break the emulsion can be evaluated.
- Make sure your extraction action on the shaker is not too severe. If so, reduce the agitation speed, not the time of extraction. The horizontal, wrist-action shaker is preferred versus the vertical clamp shakers
Zero the UV-VIS Spectrophotometer
- If using the Antifoam degas method, the spectrophotometer should be zeroed with isooctane.
Cuvettes and Glassware
- Absorbance of the sample is carried out in the ultraviolet region (100-400 nm) therefore, clean quartz cuvettes need to be used. Glass and plastic cuvettes should be avoided, as they absorb light in the ultraviolet region and will create false absorbance readings.
- All glassware should be scrupulously clean. This can be a major cause of precision difficulties. Pre-rinsing extraction glassware with small volumes of isooctane can be used to rinse cleaned cuvettes.
Thanks to our contributing Method Masters: Joe Palausky, Robert Foster, Robert Fulwiler, Jeff Petersen, Trent Leslie, Katie Fromuth (chair)
