This is the fundamental method for determining how many yeast cells is in one milliliter of sample, whether it be a yeast slurry, yeast propagation, or fermenter sample. It utilizes a tool called a hemocytometer that makes counting cells much easier due to its gridded pattern. Because of the accurate volume above the counting area, we can determine the number of yeast cells in that area of an appropriately diluted sample and extrapolate to a 1mL volume through the method calculations. This is an important value for determining pitch rate and monitoring fermentations. There are many opportunities for error therefore each step must be performed precisely and consistently
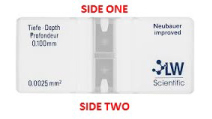
Figure 1 - Click to Enlarge
The hemocytometer is a device that uses a counting chamber with a specific volume in order to calculate the number of cells in one milliliter of a yeast sample.
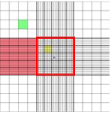
Figure 2 - Click to Enlarge
The volume of liquid directly over the ruled area of the counting chamber where yeast cells will be counted, depicted by the red square, is typically 0.0001cm3 = 1 x 10-4 cm3 = 0.0001mL or 1 x 10-4mL. This will need to be recalculated if the dimensions of the chamber are different from what is expressed in the method. The final calculations must therefore be adjusted as well.
Green Chemistry
|
Green Action |
Principle |
Benefit |
| Use EDTA as the deflocculating agent over sulfuric acid |
Safer Solvents | Prevents technicians from using a corrosive and dangerous chemical. |

Prevent Accidents
|
| Use glass volumetric pipettes for reagents transfers. |

Prevent Waste | Washing glass volumetric pipettes between each use reduces the need to single-use plastics. |
| Collect waste for proper disposal. | Working with local management for reagent disposal prevents release of hazardous materials to the environment. |
Icons provided by TheNounProject from creator iconsmind.com
A Big Thanks To...
Rob Christiansen, Liz Nagle, Krystin Norman, Joe Palausky, Robert Fulwiler, Katie Fromuth
