There are three protocols within this method: incubation, plating, and sterile membrane filtration. Each of these protocols are necessary to understand, detect and characterize growth found in samples of interest. Samples to test include (but are not limited to) process water, yeast slurries, in-process beer, and finished beer where total plate counts can be determined and contaminating microorganisms can be detected. The sample types differ in the number of cells present and the organisms of interest, therefore the appropriate techniques, incubation conditions, and media choices must be employed to achieve the desired results.
A.Incubation
Incubation is the process of growing cells to a visible level over a period of time under optimized environmental conditions for microorganisms of interest. Temperature, oxygen concentration, pH, time, and nutrient availability are examples of conditions that can be manipulated during incubation to increase the likelihood of detecting microorganisms. Incubation is carried out in an incubator at a programmed temperature. Incubators can be either aerobic (with oxygen) or anaerobic (without oxygen). Incubation under anaerobic conditions typically requires a longer incubation time to allow for sufficient growth of beer spoiling microorganisms. Another option for carrying out anaerobic incubation is with the use of oxygen scavenging sachets and an airtight chamber. pH and nutrient availability are adjusted with the media used. See Method of Analysis, Microbiological Control 4. General Culture Media, and Microbiological Control 5. Differential Culture Media to learn more about media options for brewing microorganisms.
Knowledge about the microorganisms of interest and their optimal growth conditions is necessary for setting up incubation and plating protocols appropriately to ensure accurate results. For example, to detect potential wild yeast contamination incubation should be performed on wild yeast selective media aerobically at 30ºC. To incubate anaerobically or outside the optimal temperature range of wild yeast would prevent growth and result in a false negative analysis.

Figure 1a. Example of a large incubator. Click to Enlarge. |

Figure 1b. Example of an airtight anaerobic chamber with oxygen scavenging sachet. Click to Enlarge. |
B. Plating
Plating refers to the techniques used to administer samples onto plate media and into broth media. Most plating is done with media poured into sterile Petri dishes. Petri dishes come in different sizes. The two most common sizes used in the brewing industry are 60x15-mm and 100x15-mm, with the prior being best suited for membrane filtration and the latter for pour plating, spread plating, and streaking for isolation.

Figure 2. Example of the two common sizes of Petri dishes used, 60x15mm & 100x15mm. Click to Enlarge |
Sterile membrane filtration will be expanded upon in the following section. Pour plating, spread plating, and the T-streak are three microbiological techniques frequently used in brewing microbiology labs. Pour and spread plating are used in samples expected to have high cells per milliliter of sample. Yeast slurries and beer in-process will often require dilutions to prevent overgrowth on the plate. Overgrowth is undesirable because accurate cell counts and proper examination of colonies cannot be achieved. It is best to target cell counts of 25-250 colony forming units (CFUs) for accurate results. When samples are diluted, the dilution rate must be applied to the final cell count calculation.
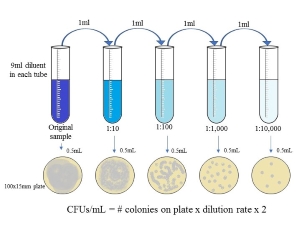
Figure 3. Diagram of creating serial dilutions from a stock liquid and the hypothetical associated growth patterns. Click to Enlarge. |
Diluents, or the liquid that the sample is being diluted into, must be sterile and osmotically favorable for microorganisms. If the osmotic pressures are unequal, microbial cells may burst which would cause cell death and inability to grow on media for detection.
The spread plate method and T-streak method is a technique used to successively spread and thin out a culture of yeast or bacteria to the point where isolated colonies grow and morphologies can be assessed to confirm the culture is pure, or to perform identification protocols (e.g., catalase test, Gram stain). It can also be used to select colonies for preservation on slants or under cryopreservation.
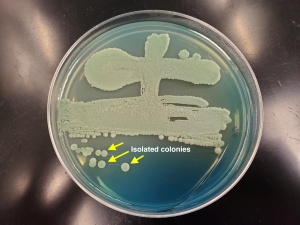
Figure 4. A WLN (100x15-mm) with an example of the "T-Streak" method for isolating colonies. Click to Enlarge. |
C. Membrane Filtration
Membrane filtration is a method used to scan a large volume of liquid sample for microorganisms. Increasing the sample volume being tested for contamination greatly increases the chance for detection of spoiling microorganisms.
The membrane filter and filter cup should be a sterile environment that allows most solubilized beer contents (e.g., sugars, chemical by-products of fermentation, non-precipitated proteins, water) to pass through the 0.45-µm pores, while catching any bacterial, yeast, and mold cells on top of the membrane filter. One way to visualize the microscopic organisms is by enumeration on agar media until a colony-forming unit (CFU) forms. Alternatively, media broths with color changing indicators are available as well. The assumption is that CFUs are isogenic (i.e., derived from a single cell), though it is possible for cells that were close together on the filter to form a mixed colony.
Membrane filtration combined with selective and differential media, and optimized incubation conditions, can help brewery microbiologists understand the microbiological content of their beers and processes, and identify potential high-risk beer-spoilage organisms.
Green Chemistry
|
Green Action |
Principle |
Benefit |
| Use clean and sterilized glass petri dishes |

Prevent Waste | Allows for multiple uses and reduces plastic waste |
| Denature cycloheximide containing media by autoclave |
Safer Solvents | Allows for cycloheximide containing media to be placed in landfill instead of hazardous waste |
| Purchase pre-made CO2 indicators |

Prevent Accidents
| Prevents technicians from creating stock solutions for CO2 indicators from hazardous chemicals |
| Use an ethanol lamp to flame loops and spreaders |

Renewable Feedstocks
| Ethanol is a renewable fuel as opposed to natural gas or propane, however there is an increased fire hazard with this action. Consult with a safety officer and/or perform hazard analysis before adopting action |
Image credit: iconsmind.com
A Big Thanks To Our Contributing Method Masters:
Krysten Norman, Liz Nagle, Michael Billon, Guy Stewart, Katie Fromuth (chair)
